but you know about photons too. You know that if something is small enough, the momentum of photons is likely to knock them about. You may even know that the resolution is restrained by the wavelength of the photon - the longer the wavelength, the less their resolving power. Think of it like pixels - the larger the pixels, the worse the image. So how do you take a photo of something very, very tiny? You use something with a shorter wavelength, of course.
Like electrons! See, you could use visible light, around 400-700nm (billionths of a meter) to see...well, all the things you see, or X-Rays, at 0.01-1nm to go through tissue and bounce off denser material such as bone. But these are still general surfaces, much too blunt for our purposes.
What's the wavelength of an electron? I'm glad you asked! To find the "Compton" wavelength of a particle you use a handy little formula and find that the electron's wavelength is about 2.43 picometers, or 0.0002nm!
Now that's a sharp image.
Tangent: did you know there are electron guns out there? PYEW!
ANYHOOS, so electrons can make out some mighty fine details indeed, and we utilize this in various ways. In the Transmission Electron Microscope, we shoot the electrons through an object, recording the diffraction (essentially the change in direction) the electrons experience and interpreting the results on the other side. This has some requirements - either the material must be very thin, or we are looking through material that is transparent in some places and opaque in others, and imaging the difference.
We also have Scanning Electron Microscopes, where instead of measuring deflection and the like of electrons on the other side of a sample, the small beam is scanned across the surface of an object (which is apparently called raster scanning), and one or more sets of emissions (secondary electrons, reflected electrons, characteristic x-rays, etc.) are recorded in detectors placed in useful positions. This results in a slightly lower resolution, but allows one to image thicker objects. Whenever you see a highly detailed image of an insect or some such floating around, that's usually from an SEM (coated in a substance like gold which makes the whole process extraordinarily simpler).
One of our cleverest imaging techniques involves the very peculiar quantum tunneling effect. As a quick summary: our classical view of particles is that if a particle encounters an infinitely strong barrier, it bounces off. Since, as we know now, there is a wave-particle duality, this is no long exactly true. The wavefunction of the particle will not just be reflected, but it will exponentially decay in the presence of the barrier. If the barrier is thin enough that the wavefunction does not decay completely to zero by the time it reaches the other side, there is a statistical chance of the particle getting through. I mentioned this indirectly as the cause of fusion in stars.
Basically, the Scanning Tunneling Microscope involves getting a very fine tip near a surface with a potential difference between them which causes a slight current between the two (the 'barrier' being tunneled through here is the empty vacuum between them). Changes in this current correspond to changes in distance to the surface, so that as it scans across, a full image can be generated.
There's another, very precise method, using Atomic Force Microscopes, which I've learned about only recently. Instead of generating a current between the tip and a sample, the tip is moved close enough that it actually feels various forces that push and pull on it ever so delicately. (very general description)
All that was the long road to the point of this post, which is right here:
That, my friends, is an insanely detailed image of graphene, a one-atom thick sheet of carbon in a hexagonal pattern. So detailed, in fact, that never-before-seen differences and strengths of chemical bonds can be rendered.
They did this by taking the AFM technique a step further: They used the tip to grab onto a single carbon-oxygen molecule, and then used that to probe the atomic surface. They had to keep everything so free of vibrations, it was cooled down to -268 Celsius (5.15 Kelvin), which is only twice the temperature of the cosmic microwave background, the remnant heat from the big bang!
This is truly peeking into the inner workings of molecules with what amounts to the finest needle physically possible. We may even be able to manipulate the atoms within molecules with this technique. Very cool.
Like electrons! See, you could use visible light, around 400-700nm (billionths of a meter) to see...well, all the things you see, or X-Rays, at 0.01-1nm to go through tissue and bounce off denser material such as bone. But these are still general surfaces, much too blunt for our purposes.
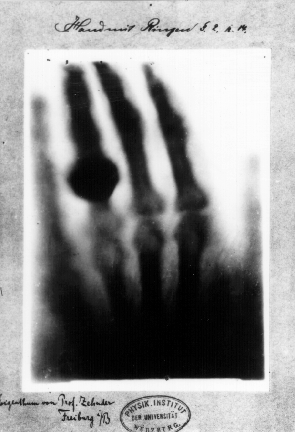 |
| First "medical" X-Ray image: Roentgen's WIFE'S hand. Not what I would call chivalrous. (wiki) |
What's the wavelength of an electron? I'm glad you asked! To find the "Compton" wavelength of a particle you use a handy little formula and find that the electron's wavelength is about 2.43 picometers, or 0.0002nm!
Now that's a sharp image.
Tangent: did you know there are electron guns out there? PYEW!
ANYHOOS, so electrons can make out some mighty fine details indeed, and we utilize this in various ways. In the Transmission Electron Microscope, we shoot the electrons through an object, recording the diffraction (essentially the change in direction) the electrons experience and interpreting the results on the other side. This has some requirements - either the material must be very thin, or we are looking through material that is transparent in some places and opaque in others, and imaging the difference.
We also have Scanning Electron Microscopes, where instead of measuring deflection and the like of electrons on the other side of a sample, the small beam is scanned across the surface of an object (which is apparently called raster scanning), and one or more sets of emissions (secondary electrons, reflected electrons, characteristic x-rays, etc.) are recorded in detectors placed in useful positions. This results in a slightly lower resolution, but allows one to image thicker objects. Whenever you see a highly detailed image of an insect or some such floating around, that's usually from an SEM (coated in a substance like gold which makes the whole process extraordinarily simpler).
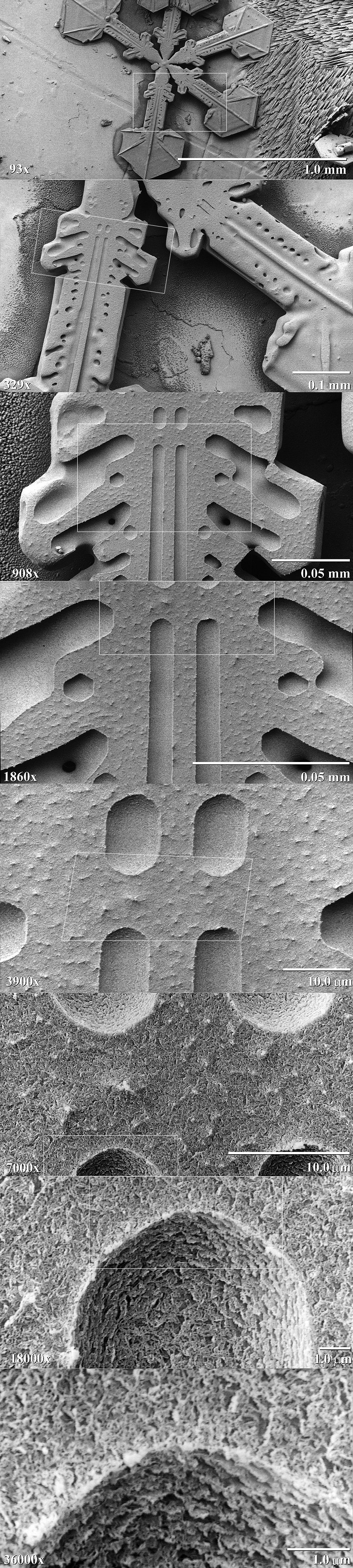 |
| Take a snowflake, coat it in platinum, zoom in. Yes, I said coat the snowflake in platinum. (wiki) |
One of our cleverest imaging techniques involves the very peculiar quantum tunneling effect. As a quick summary: our classical view of particles is that if a particle encounters an infinitely strong barrier, it bounces off. Since, as we know now, there is a wave-particle duality, this is no long exactly true. The wavefunction of the particle will not just be reflected, but it will exponentially decay in the presence of the barrier. If the barrier is thin enough that the wavefunction does not decay completely to zero by the time it reaches the other side, there is a statistical chance of the particle getting through. I mentioned this indirectly as the cause of fusion in stars.
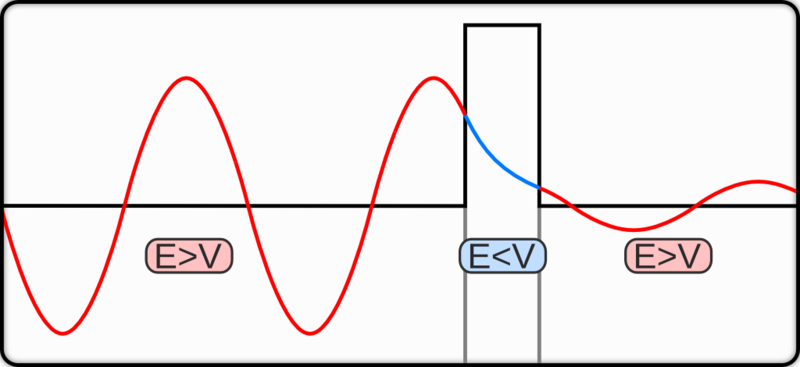 |
| (Wiki) |
There's another, very precise method, using Atomic Force Microscopes, which I've learned about only recently. Instead of generating a current between the tip and a sample, the tip is moved close enough that it actually feels various forces that push and pull on it ever so delicately. (very general description)
All that was the long road to the point of this post, which is right here:
 |
| BBCNews |
They did this by taking the AFM technique a step further: They used the tip to grab onto a single carbon-oxygen molecule, and then used that to probe the atomic surface. They had to keep everything so free of vibrations, it was cooled down to -268 Celsius (5.15 Kelvin), which is only twice the temperature of the cosmic microwave background, the remnant heat from the big bang!
This is truly peeking into the inner workings of molecules with what amounts to the finest needle physically possible. We may even be able to manipulate the atoms within molecules with this technique. Very cool.
No comments:
Post a Comment