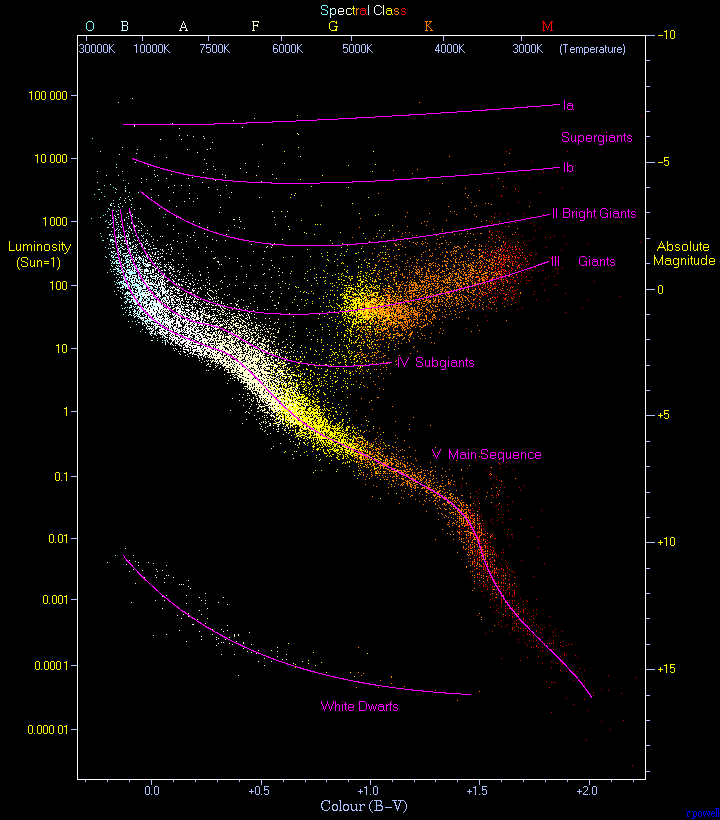
One of the nice things about stars is that they are, in their own way, fairly simple things. Just a bunch of Hydrogen, some Helium, trace amounts of other elements, mushed together by gravity so powerfully that they ignited. Their general behavior is almost entire defined by the amount and proportion of their chemical makeup. And there's so very many stars knockin' about, we've cataloged enough to present a decent continuum of possible states.
(23,000 stars plotted on an HR Diagram)
Another nice thing about stars is that we can basically see their insides, via the electromagnetic energy pouring out of the surface. This energy is characteristic of the reactions taking place. Spectroscopy studies this relationship between matter and photons.
There are three ways matter emits light that are of interest to scientists. One is the common black body radiation, based on the temperature of very hot things (when you see heated objects turn red, this is black body radiation). Because they're all jostling about, it forms a continuous spectrum. The second is emission spectra, which peak at wavelengths characteristic of the chemical reactions/electron excitations/ionizations. The third, the converse of emission, is absorption. When a continuous spectrum of light falls on some cool and mostly transparent gas, the gas can absorb specific wavelengths of light, leaving a vacancy in the continuity. Thus, the emission spectrum of a material has bright lines in the same place that it's absorption spectrum (with a continuous background) would have dark lines.
These are actually known as Kirchhoff's laws:
- An incandescent solid, liquid, or gas under high pressure emits a continuous spectrum.
- A hot gas under low pressure emits a "bright-line" or emission-line spectrum.
- A continuous spectrum source viewed through a cool, low-density gas produces an absorption-line spectrum.
Spectroscopy is the analysis of these laws; the light is sent through a diffraction grating, spreading it's differing wavelengths. Spectral analysis was already happening with materials on Earth for a few decades, but in the late 1800's Kirchhoff had the clever idea of aiming that analysis upwards. For the first time humanity knew that the objects up there were made of the same material as objects down here. As a quick example lets look at Helium, which was actually discovered in our sun 14 years before it was discovered on Earth, even deriving its name from Helios, Greek mythology's personification of the sun.
Helium Spectrum
Hydrogen Spectrum
Now, with quantum mechanics, we can understand the existence of these characteristic lines in terms of an electron raising or lowering its energy state.
So using spectroscopy, we can understand the composition of a star. We can know the temperature, by its black body radiation, and we know how much energy it takes to, say, ionize Hydrogen, so we know what's going on if we see that spectral line. Looking back up top, you can see how the cooler stars (O, B, A...) have two Hydrogen absorption lines (corresponding to the Balmer series), which imply that Hydrogen in its second excited state is absorbing photons. The hotter stars indicate the existence of Helium with it's characteristic yellow line. And this is all just along the range we're lucky enough to spot with our eyes. This analysis can take place across the entire electromagnetic spectrum.
It's almost too mad to consider: we can determine the chemical make-up of things we'll never go near, just by the information it beams outward - a story told in all directions of it's internal structure, so long as you're (scientifically) literate enough to read it.


No comments:
Post a Comment